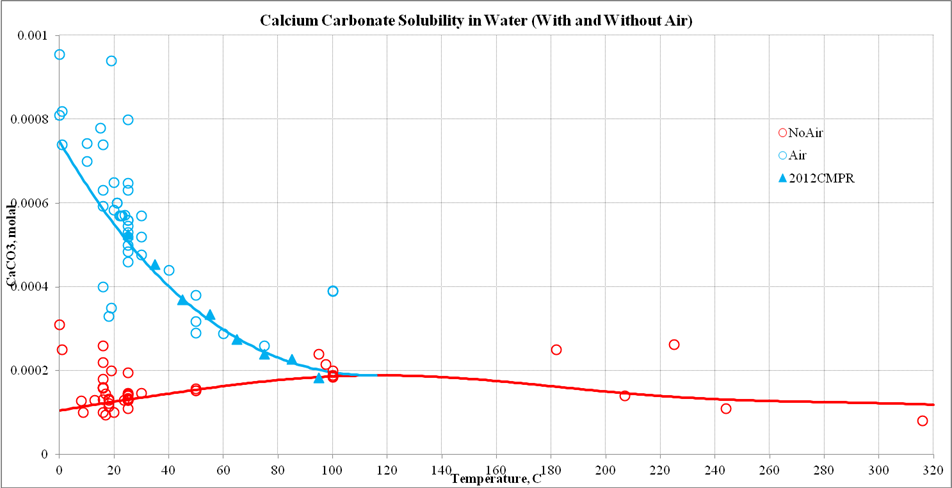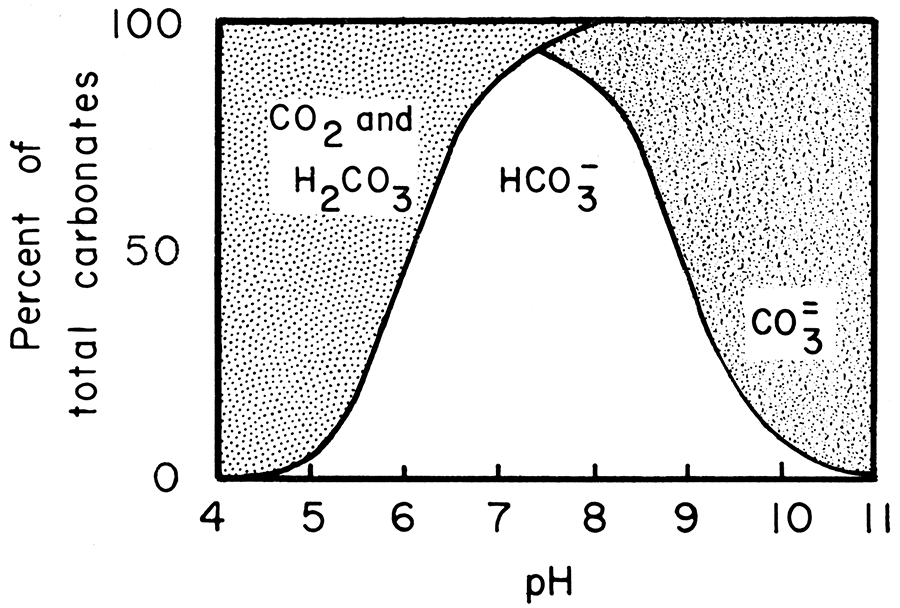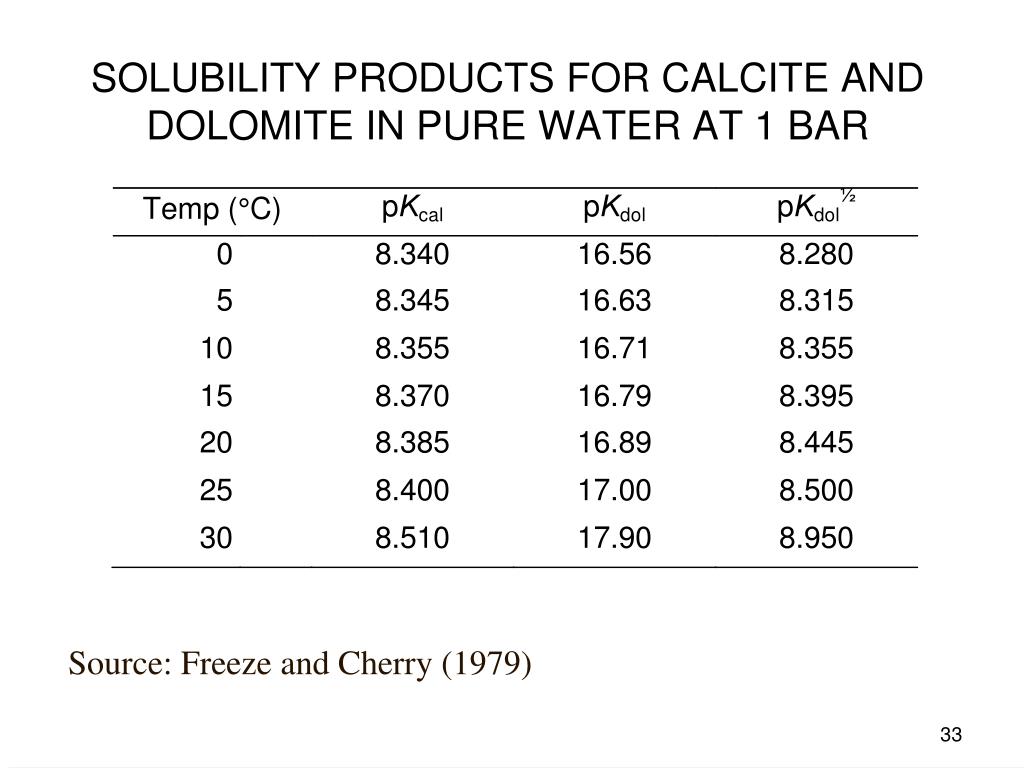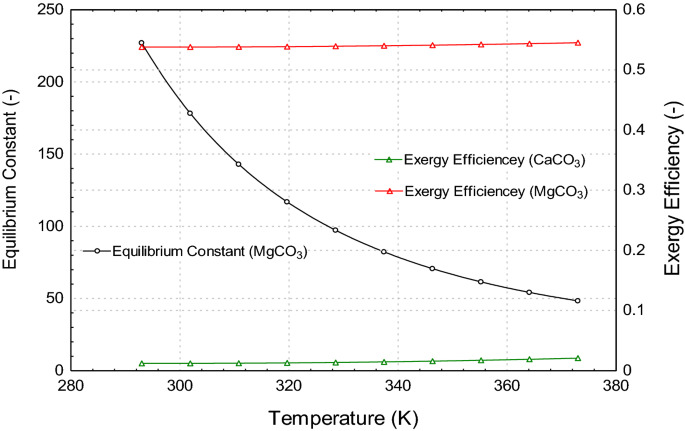
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink
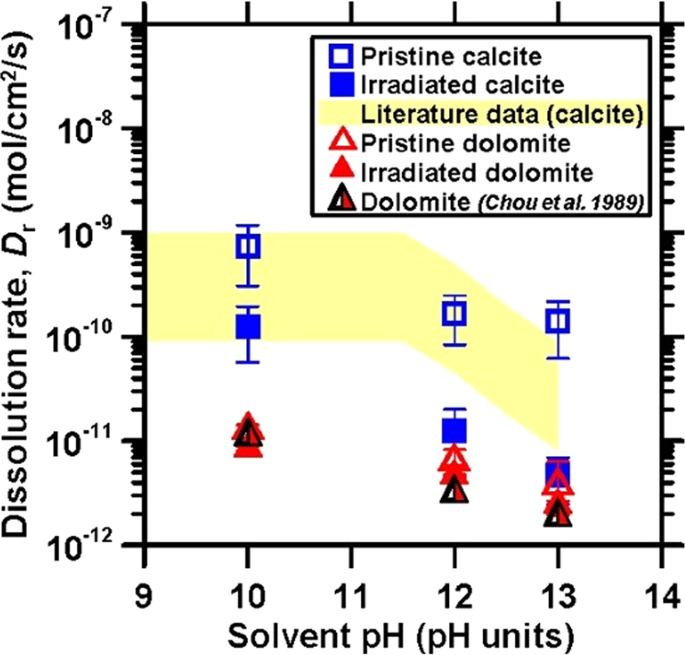
The effect of irradiation on the atomic structure and chemical durability of calcite and dolomite | npj Materials Degradation

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

An experimental study simulating the dissolution of gypsum rock - Dongdong Hong, Ming Fan, Lingjie Yu, Jian Cao, 2018

The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines - ScienceDirect

Self-accelerating volumetric dolomite-for-calcite replacement: A possible mechanism for high-temperature dolomitization? | SpringerLink
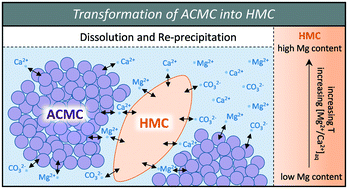
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite - CrystEngComm (RSC Publishing)
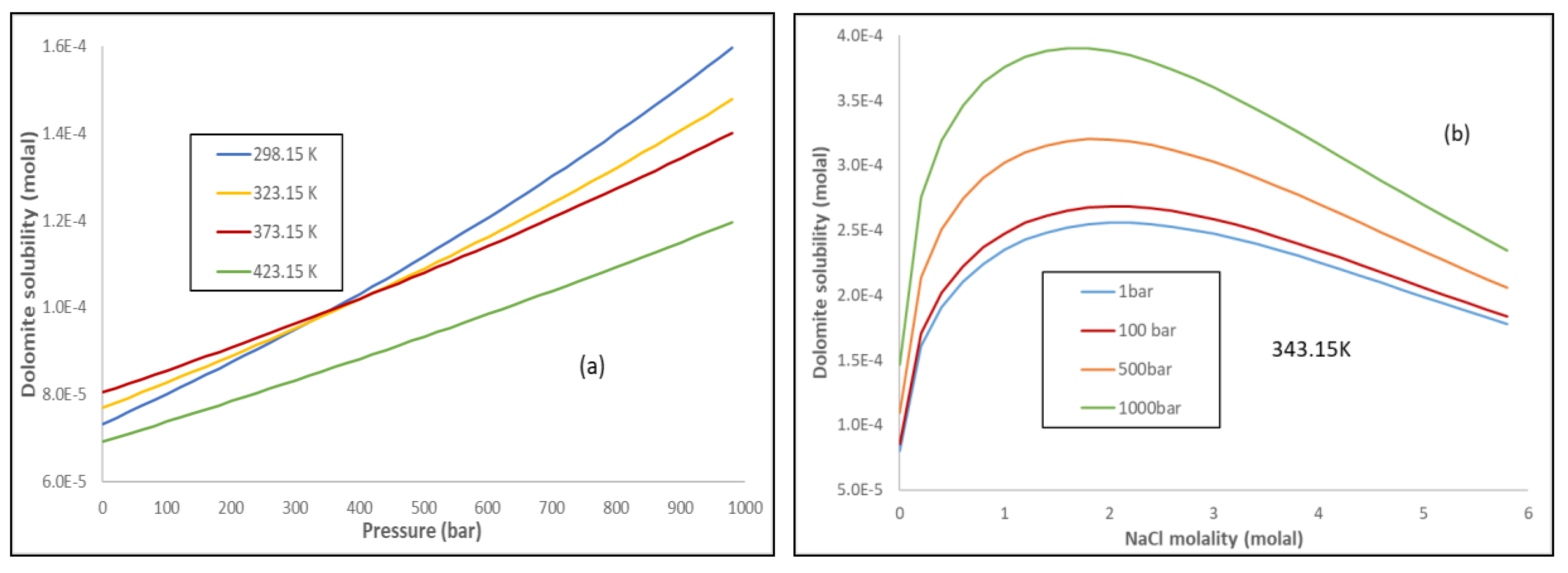
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

5 Temperature Control of Mineral Deposition – A Conceptual Overview of Surface and Near Surface Brines and Evaporite Minerals