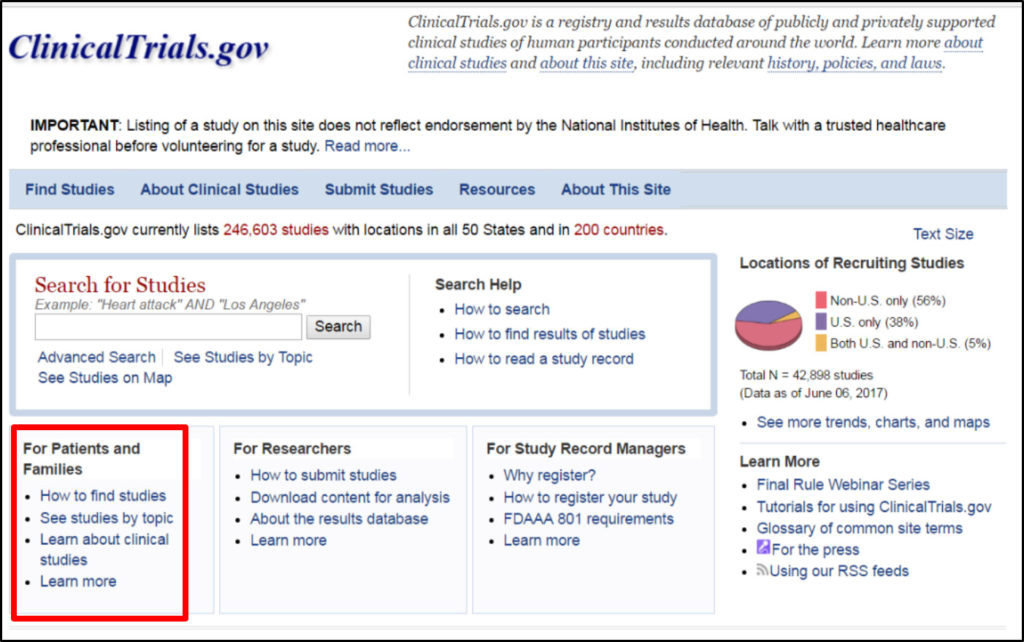
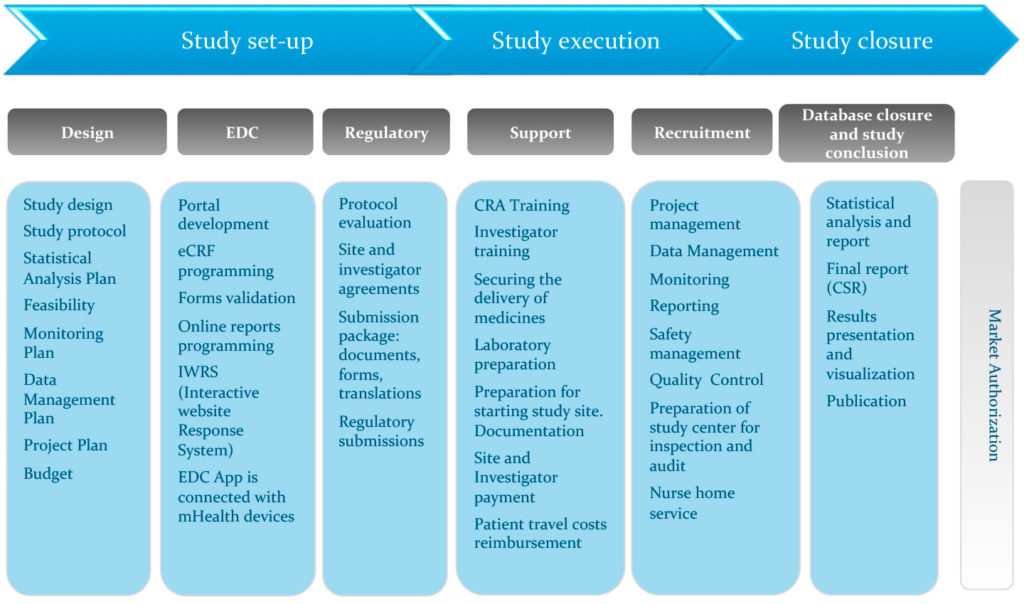
Atlanta Pediatric Research | NIH Requirements for Human Subject Research | Clinical Research Resources | Research Resources | Research | Emory + Children's + GT | Atlanta Pediatric Research Alliance

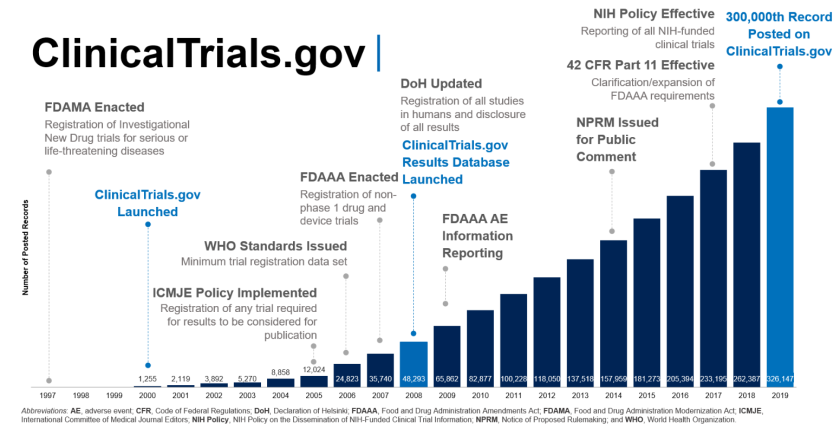
Final Rule Confirms, Posting of Study Results on ClinicalTrials.gov Will be Required for Unapproved Products - IMPACT Pharmaceutical Services, Inc.
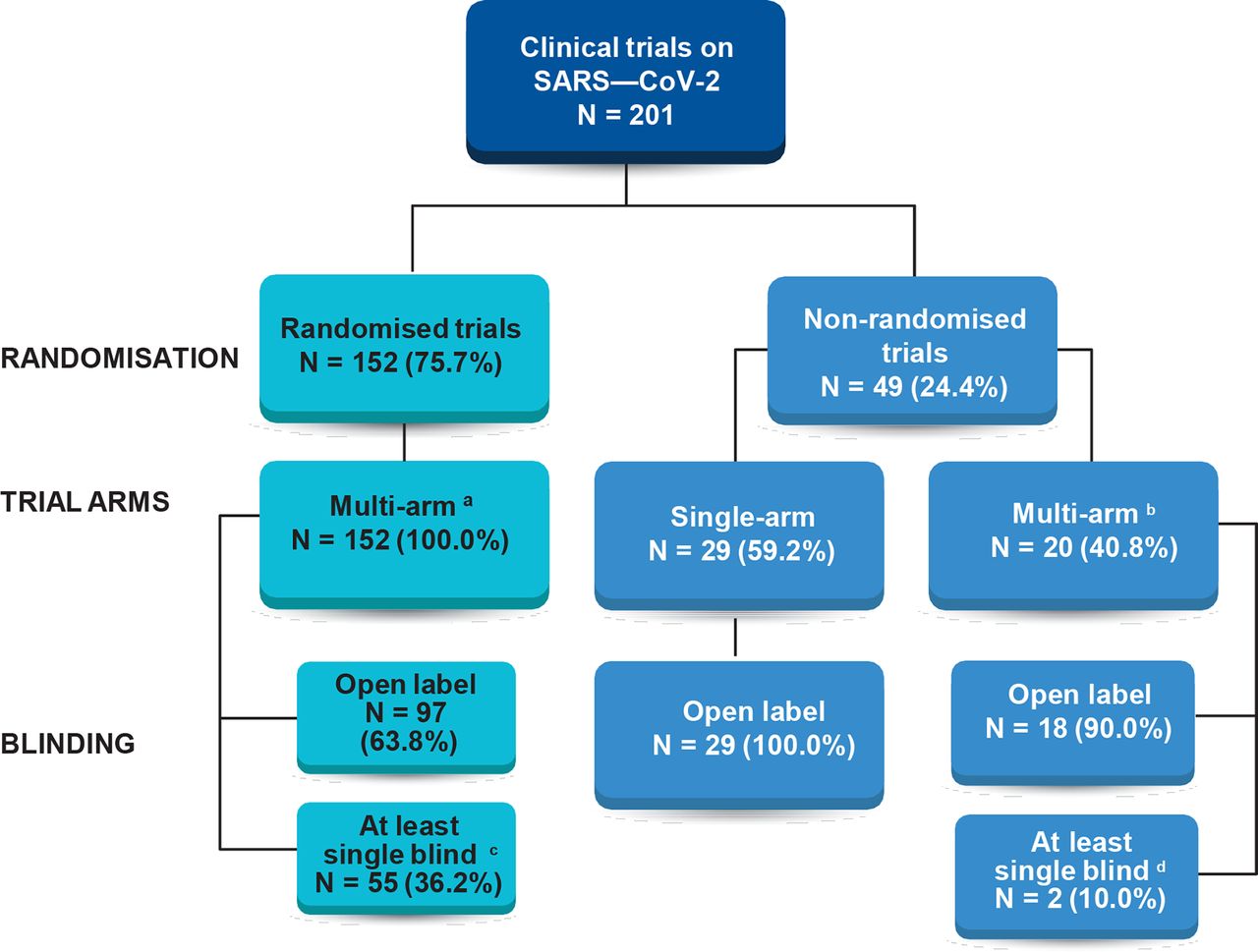
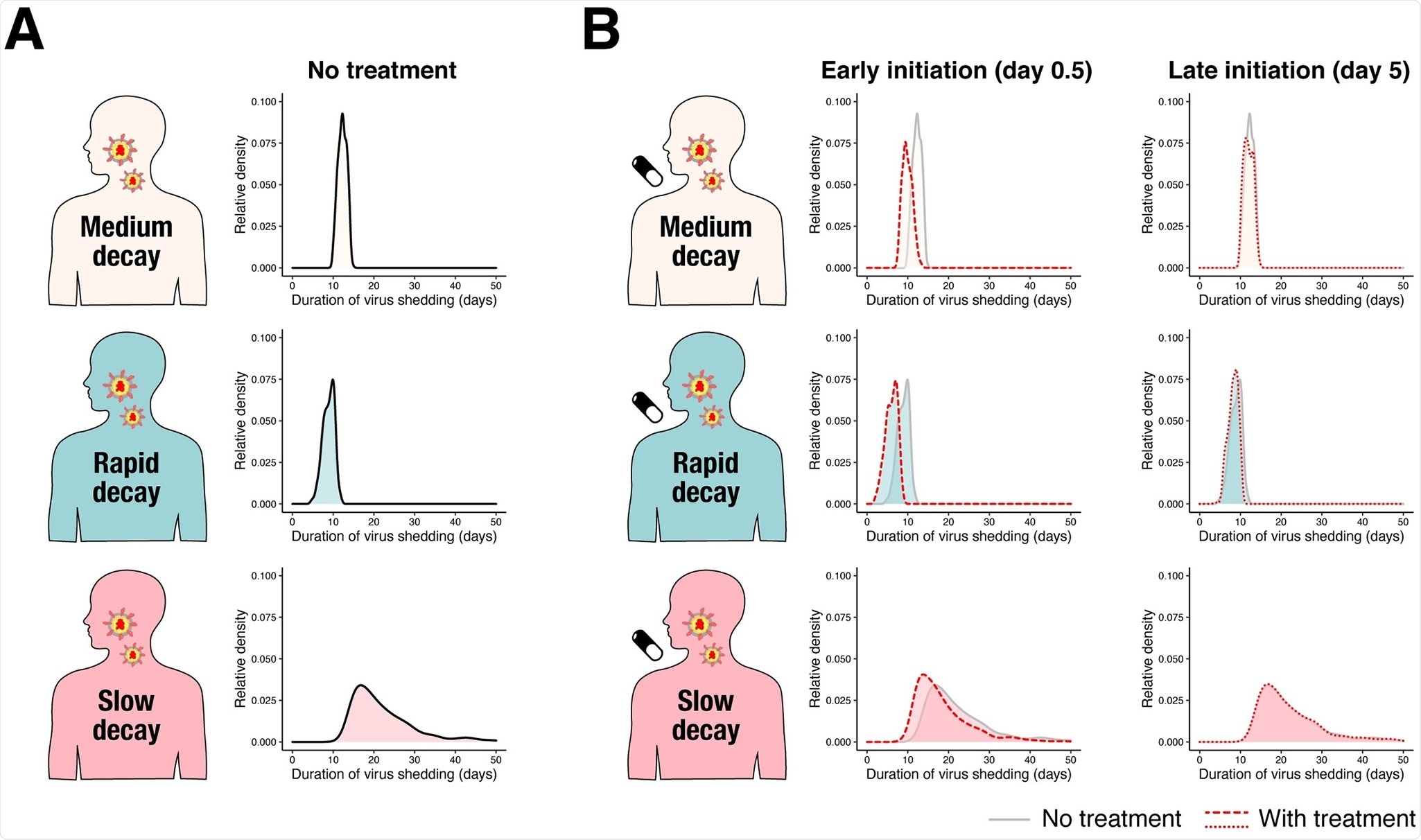
Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis | BMJ Open
How Frequently Do the Results from Completed US Clinical Trials Enter the Public Domain? - A Statistical Analysis of the ClinicalTrials.gov Database | PLOS ONE
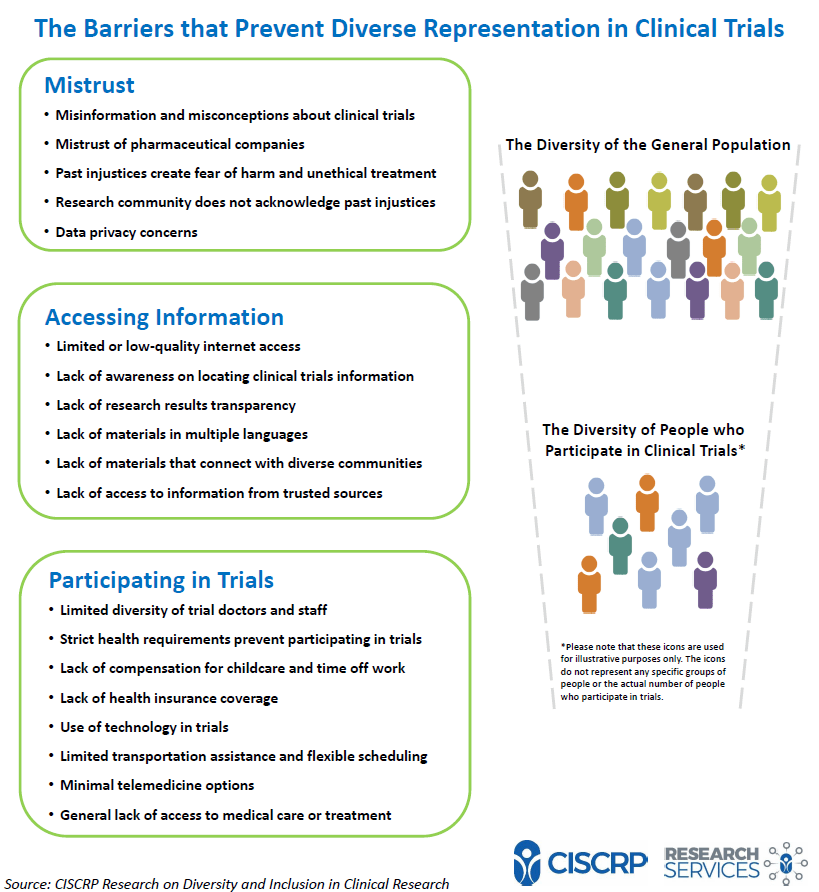
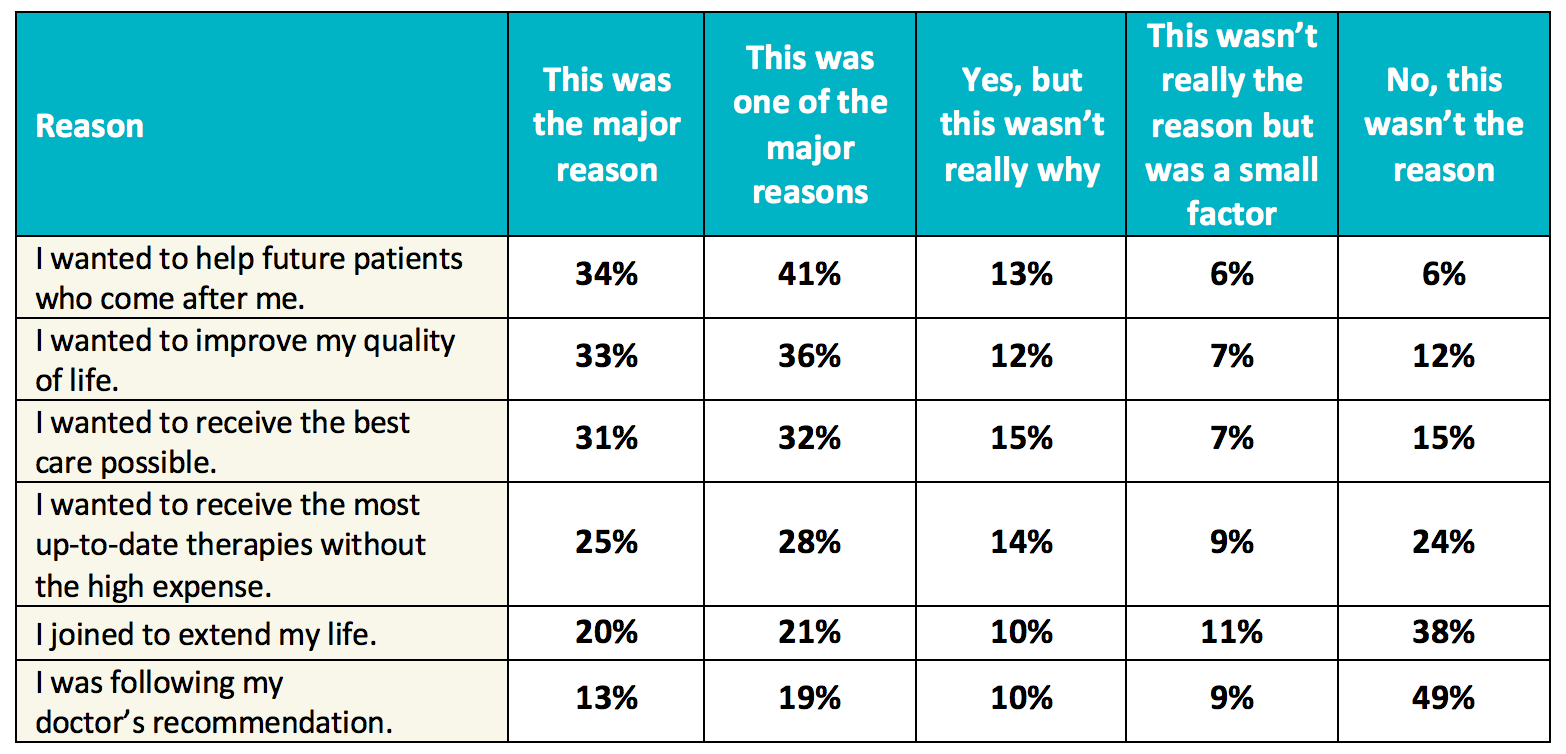
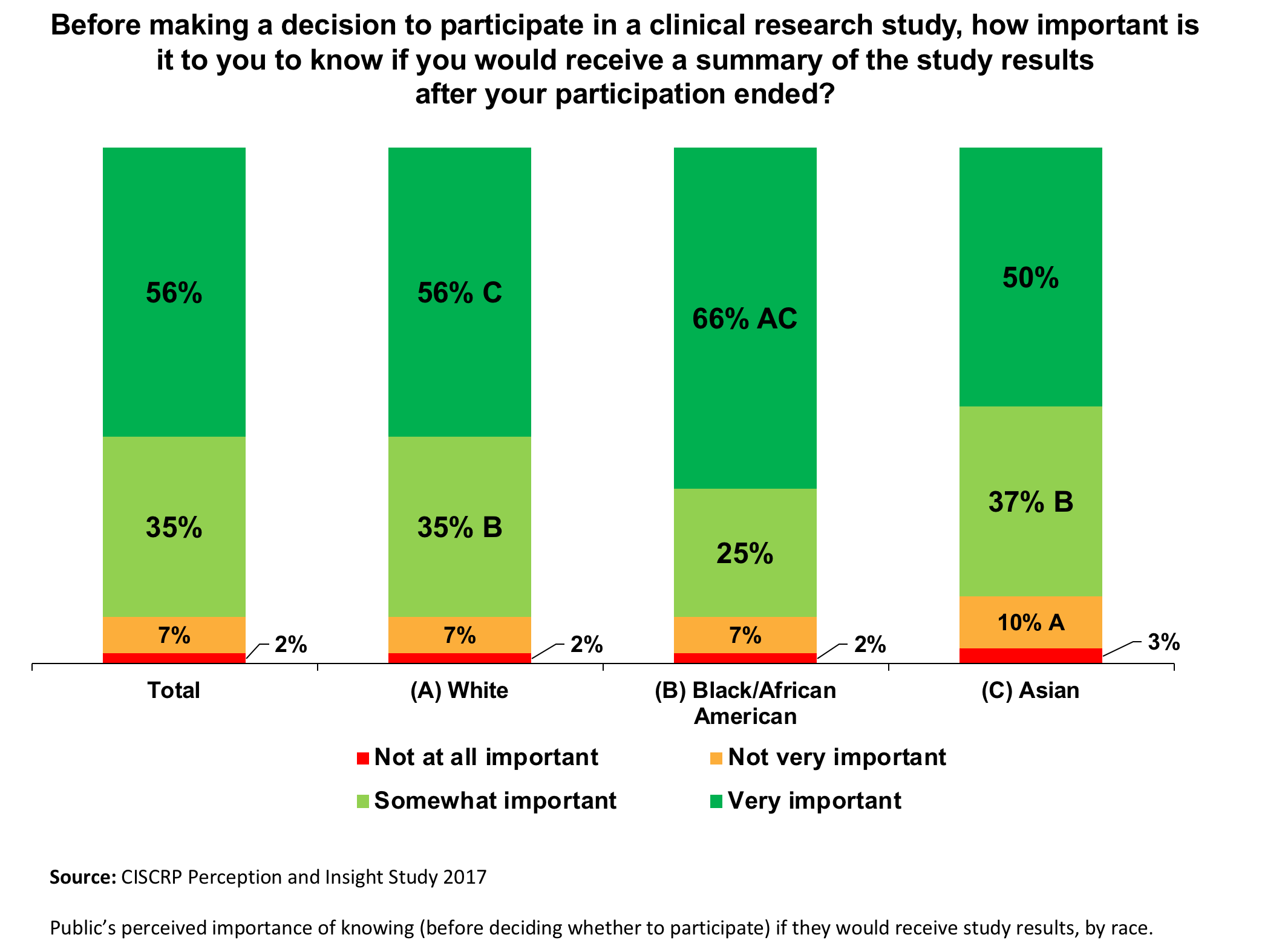
Doing Our Part: Improving Diversity in Clinical Research Participation - Center for Information & Study on Clinical Research Participation
Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials | PLOS Medicine
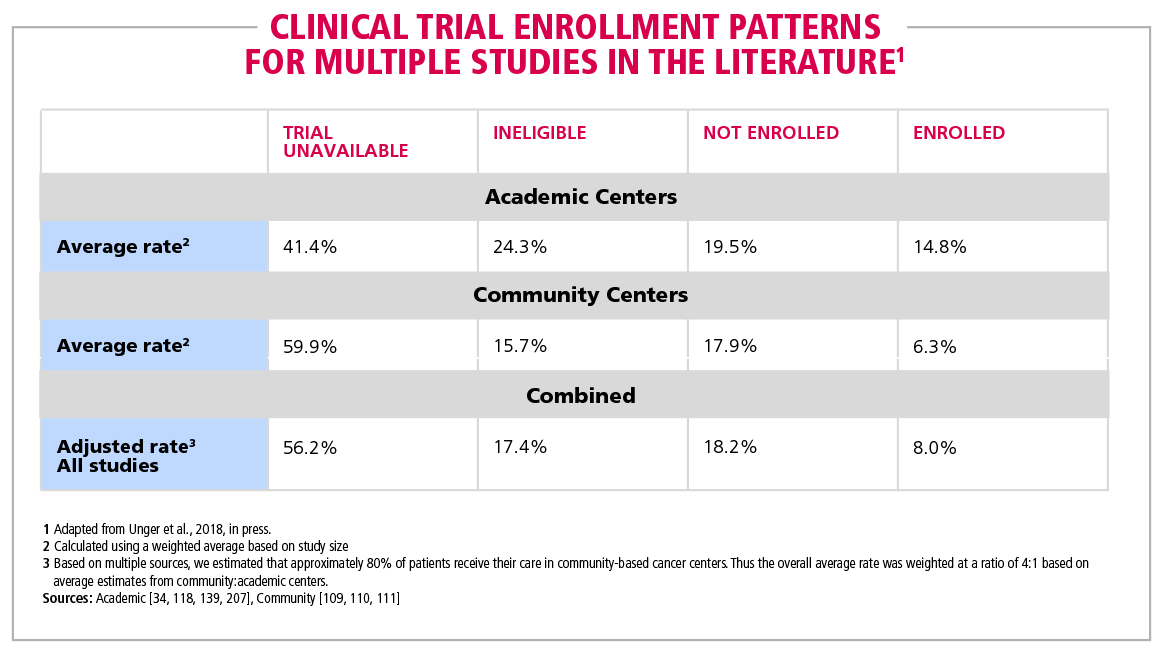
Table 1. Clinical trial enrollment patterns for multiple studies in the literature | American Cancer Society Cancer Action Network

Clinical Trials - Grey Literature in the Health Sciences - Research Guides at University of Alabama - Birmingham
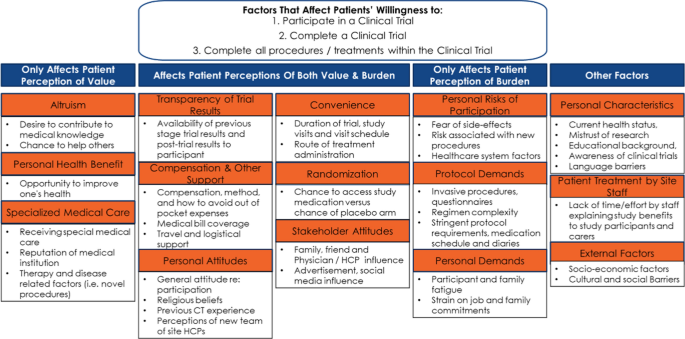
Amplifying the Voice of the Patient in Clinical Research: Development of Toolkits for Use in Designing and Conducting Patient-Centered Clinical Studies | SpringerLink