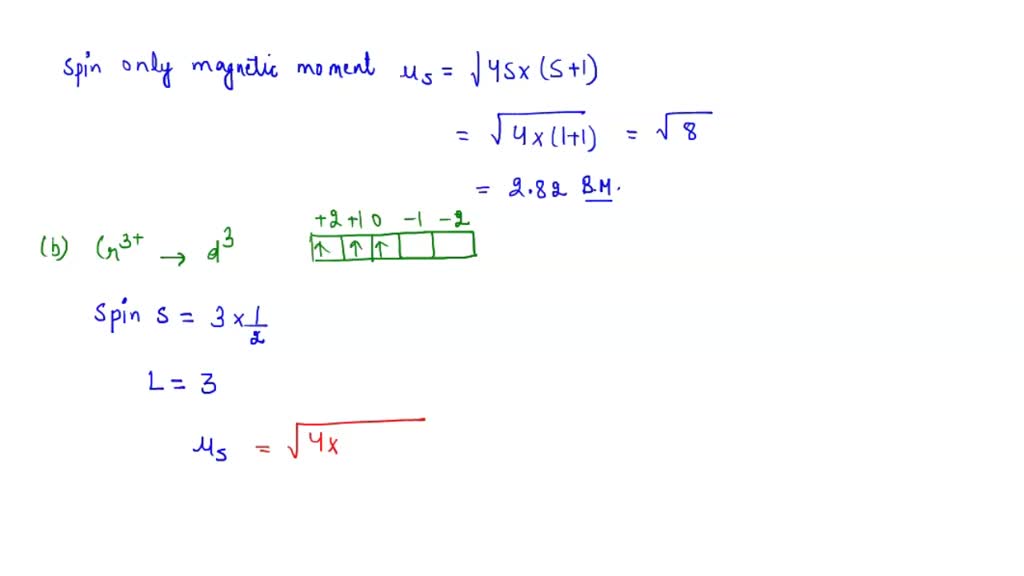
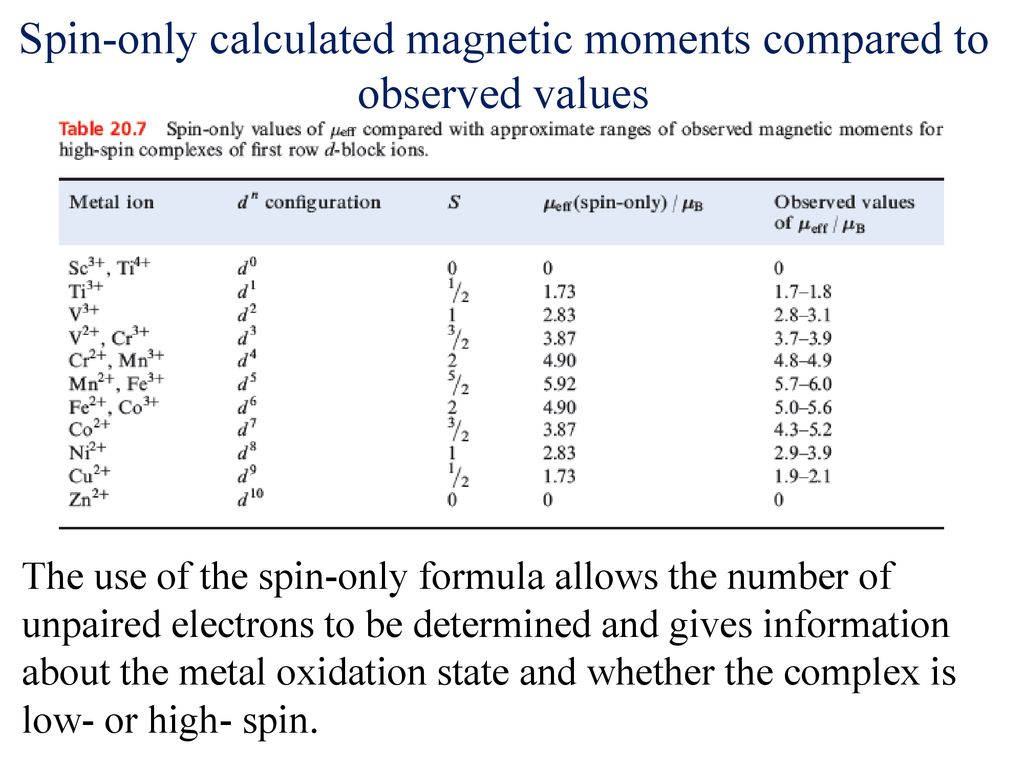
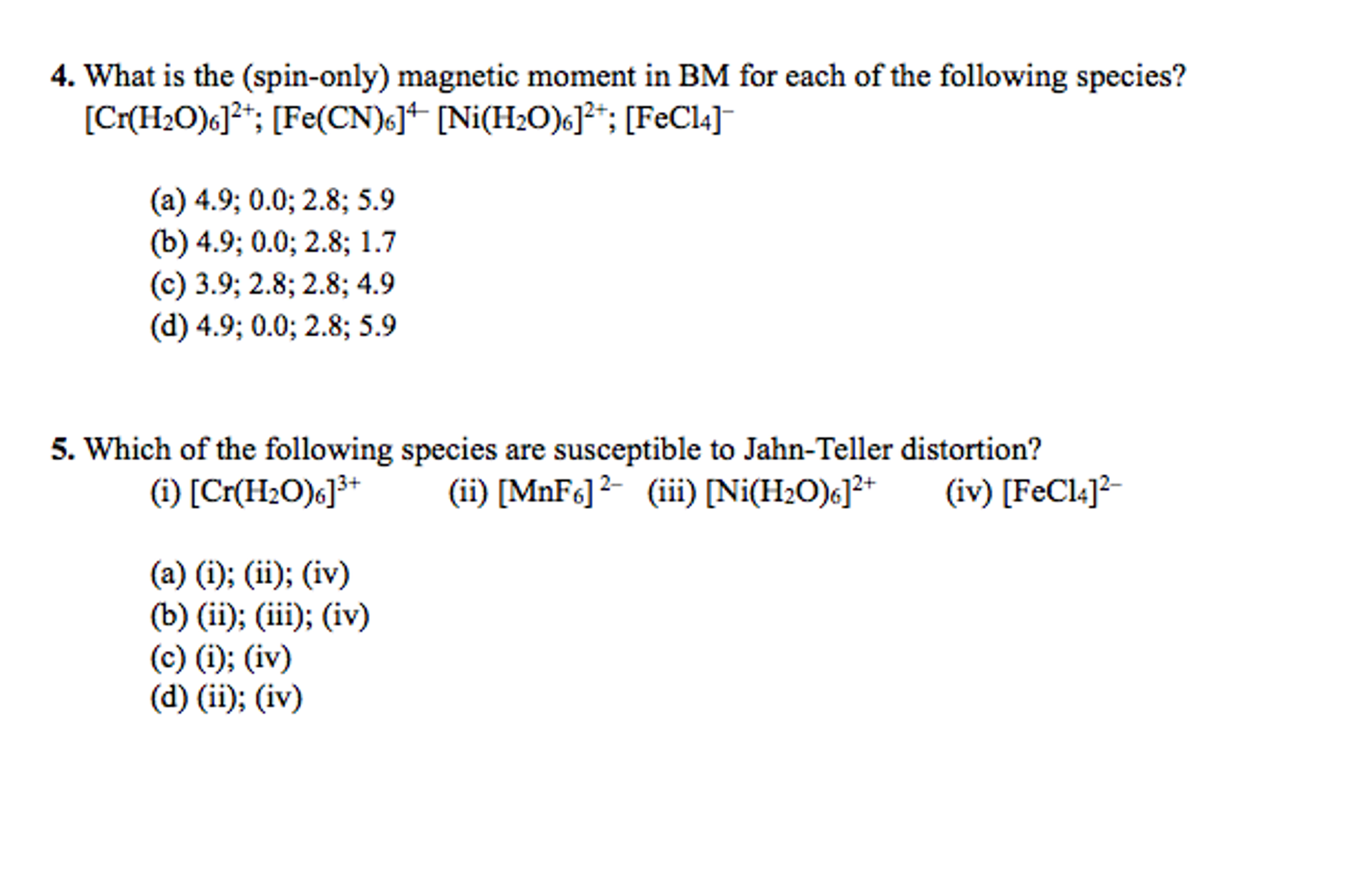
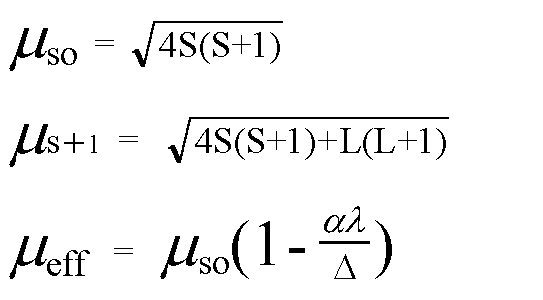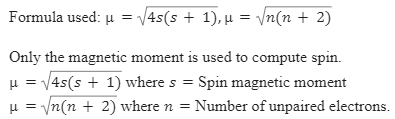The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 BM. The correct one is - Sarthaks eConnect | Largest Online Education Community

Solve this: Q 85 The spin only magnetic moment value ( in B M units) of CrCO6 is - Chemistry - The d-and f-Block Elements - 12561515 | Meritnation.com

Spin only magnetic moment of `._(25)Mn^(x+)`ion is `sqrt(15)` B.M. Then, the value of `x` is `:` - YouTube

The highest value of the calculated spin-only magnetic moment (in BM) among all the transition - YouTube
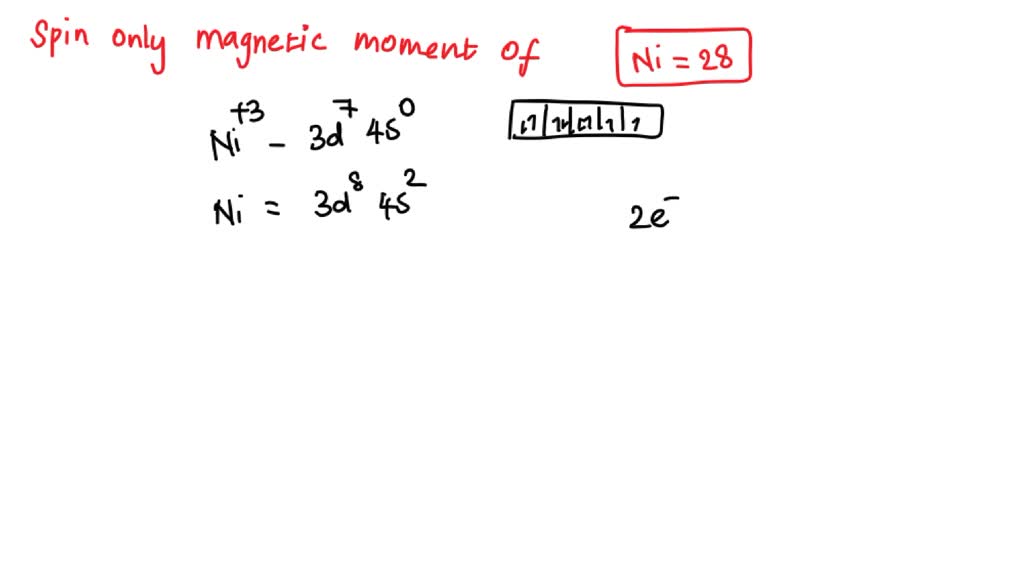
SOLVED: What is value of spin only magnetic moment of Ni (Z=28) in +3 oxidation state ? a)2.8 BM b) 3.1 BM c)0.0 BM d) 1.7 BM
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)
The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube
![The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/618499.jpg)
The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are