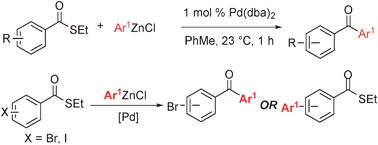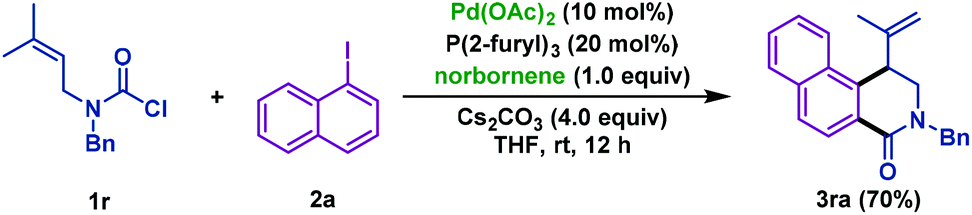
Pd/NBE-catalyzed sequential carbamoylation/olefination of aryl iodides - Organic Chemistry Frontiers (RSC Publishing)
![5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/01/5518-52-5.png)
5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation
The preparation, resolution and application of novel 2-furyl phosphine ligands in asymmetric synthesis
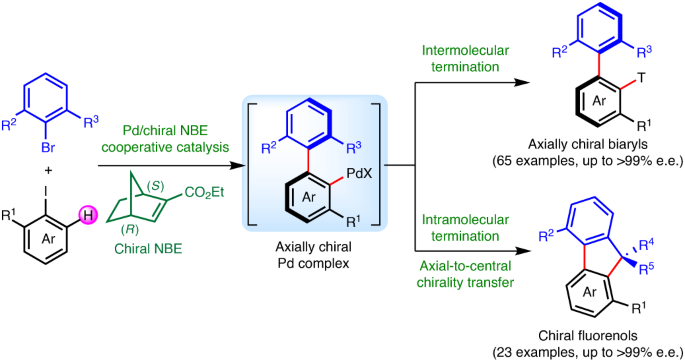
Construction of axial chirality via palladium/chiral norbornene cooperative catalysis | Nature Catalysis
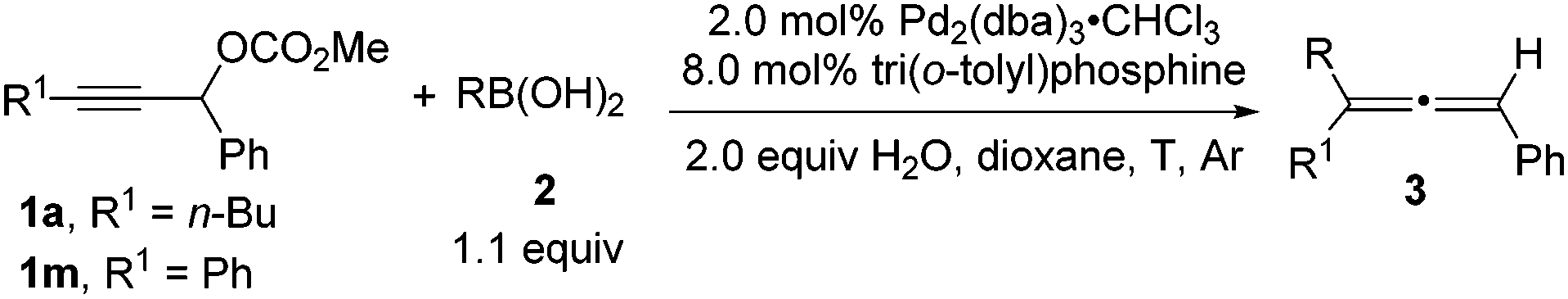
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect

CHAPTER 1 New Chemistry for Organic Photovoltaic Materials (RSC Publishing) DOI:10.1039/9781782622307-00001

Activation of AllylUE (1) and PropUE (2) probes in presence of Pd or Pt... | Download Scientific Diagram

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect
![Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/4dbbd39f-04c1-40c9-be16-0a73bbc6236d/mgra001.jpg)
Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library

Cationic palladium(II)–acetylacetonate complexes containing phosphine and aminophosphine ligands and their catalytic activities in telomerization of 1,3-butadiene with methanol - ScienceDirect
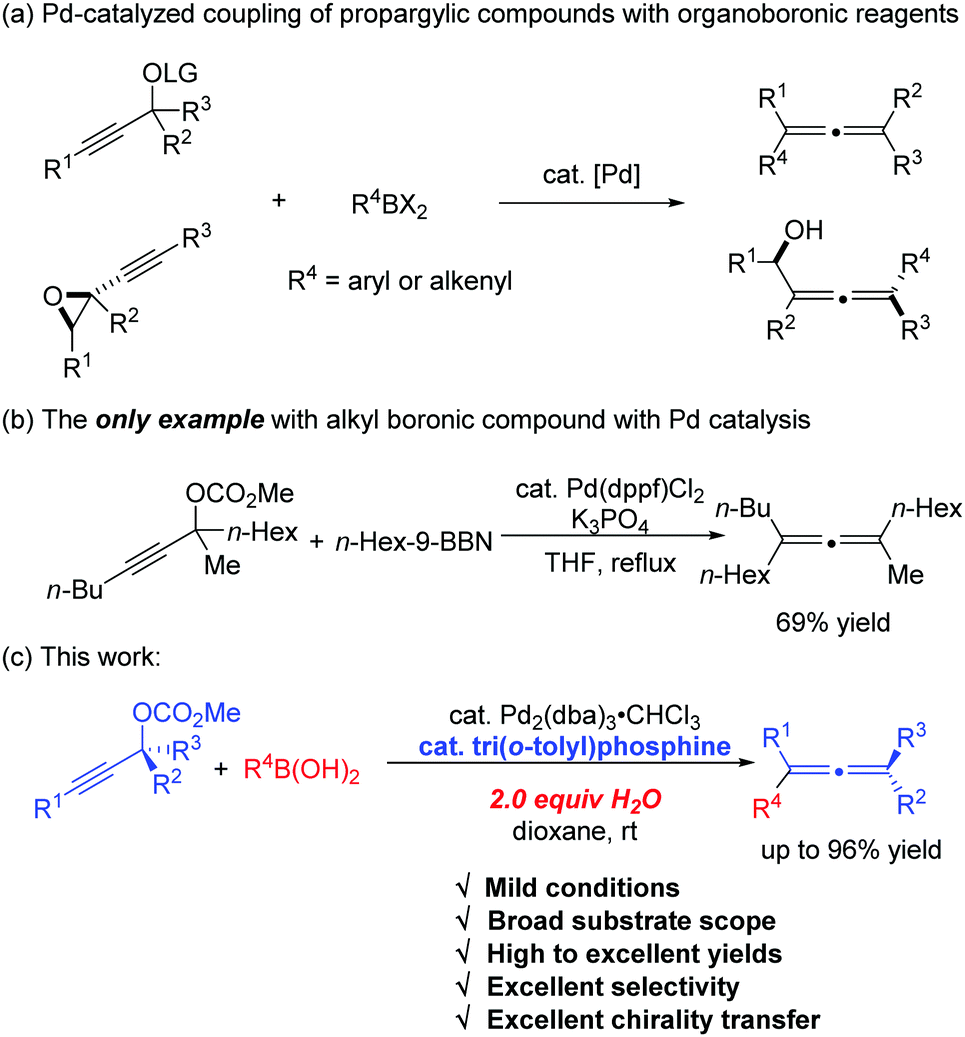
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)

Convenient and General Palladium‐Catalyzed Carbonylative Sonogashira Coupling of Aryl Amines - Wu - 2011 - Angewandte Chemie International Edition - Wiley Online Library